This post explores the National Pharmaceutical Pricing Authority’s powers of price control and monitoring on the Indian medical device industry against the backdrop of India’s drive towards an ‘Atmanirbhar Bharat.’
Introduction:
There are many barriers to affordable healthcare in India[1]. Several initiatives and mechanisms have been suggested over the years, to tackle this. One mechanism is the National Pharmaceutical Pricing Authority’s (‘Pricing Authority’) powers of price monitoring and control. This article explores what the Pricing Authority’s powers mean for the medical device industry.
An Overview of Drug Price Control:
The rationale for price monitoring and control is to keep profiteering in check and to increase access to affordable healthcare. Periodically, a National List of Essential Medicines (‘Essential Medicines List’) is compiled after stakeholder and inter-ministerial consultation. The Essential Medicines List helps the government decide the pharmaceutical products it should consider for price control and monitoring. The Pricing Authority[2] has been tasked[3] to implement price monitoring and price control through Drug Price Control Orders[4], amended from time to time.
The functioning of the National Pharmaceutical Pricing Authority:
The latest edition of the Drug Price Control Order was issued in 2013[5]. The 2013 edition lists several powers of the Pricing Authority. It also contains an Essential Medicines List[6] that lists drugs, active pharmaceutical ingredients, and drug formulations. The Drug Price Control Order also gives the Pricing Authority the power to monitor drugs that are not in the Essential Medicines List.
To decide pricing, price control, and price monitoring, the Pricing Authority relies on inputs from the Government, market surveys, and industry (through the periodic submission of the information listed below, and other communications). The graphic below depicts how the Pricing Authority exercises its powers:
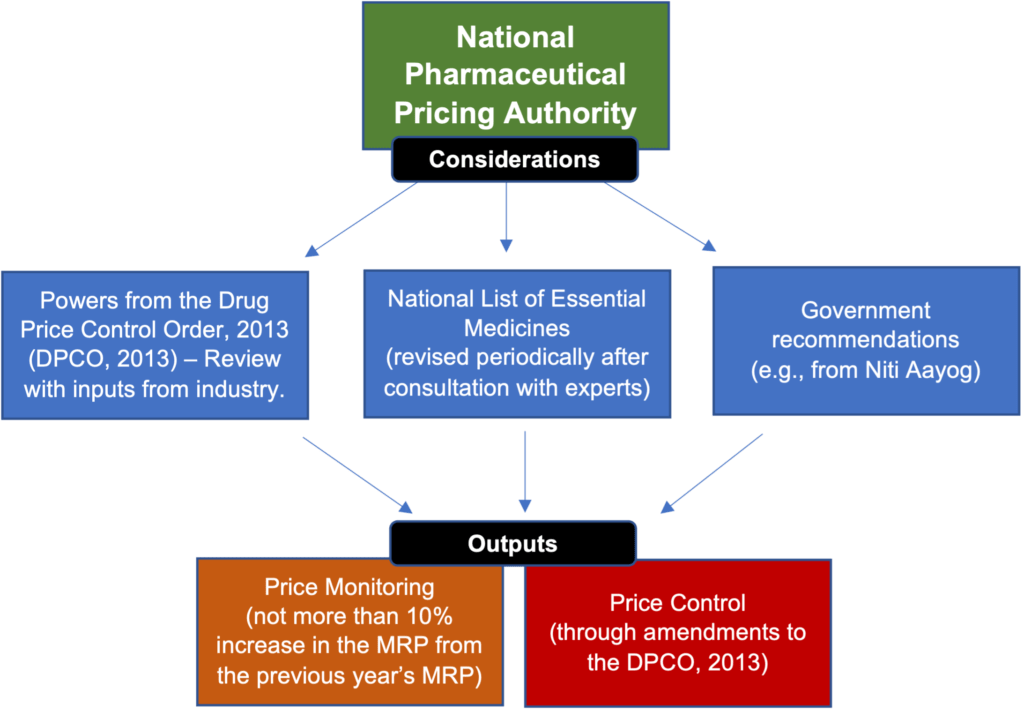
The table below describes the powers of the Pricing Authority:
| No. | Power | Description |
| 1 | Calculation of prices | Calculating the prices of: · Drug formulations[7] (i.e., a medicine processed or containing one or more drugs[8]). · Active pharmaceutical ingredients (i.e., any pharmaceutical, chemical, biological or plant product used in a formulation)[9]. |
| 2 | Control of Prices | The Pricing Authority can put a cap on the prices of drug formulations in case of the absence of price reduction due to the absence of competition[10]. |
| 3 | Notify DFs and APIs | Notify active pharmaceutical ingredients and drug formulations – even if they do not feature in the Essential Medicines List – in the public interest[11]. |
| 4 | Price Monitoring | The Pricing Authority can monitor the Maximum Retail Price (‘MRP’) of all active pharmaceutical ingredients and drug formulations, whether or not they are listed in the Essential Medicines List. This is where the Pricing Authority derives its powers of oversight of medical devices[12]. The Pricing Authority ensures that manufacturers and importers do not increase the MRP more than 10% of the MRP of the previous year, through its price monitoring functions. Where an increase is made beyond 10%, the Pricing Authority has the power to reduce it for the following year [13]. The Pricing Authority can recover overcharged amounts[14]. |
| 5 | Monitoring availability of Listed drug formulations | The Pricing Authority will monitor the availability of these scheduled drug formulations and active pharmaceutical ingredients. Their manufacturers and importers must report the following information quarterly[15]: · Name and address of the manufacturer/importer; · Name and address of marketing company, if any, and; · Details of production/import and sale for each quarter of the year[16]. |
| 6 | Call for records and conduct inspections | Manufacturers and importers must maintain records on the sale of active pharmaceutical ingredients and drug formulations. The Pricing Authority can request these records periodically[17]. |
| 7 | Segments exempt from Pricing Authority oversight | In 2019, the Department of Pharmaceuticals amended the Drug Price Control Order, 2013 to exclude the following types of drugs from price control and price monitoring: · Drugs for treating orphan diseases (i.e., diseases affecting less than five-lakh people in India[18]); · A five-year exemption from price control and monitoring for manufacturers of a ‘new drug’ (a drug that has not been used significantly in India, or is being proposed for use with new dosages and indications; or a vaccine, gene therapy, or novel delivery system for the drug[19]) patented under Indian patent laws[20]. |
Medical Devices and the Pricing Authority:
The Past: how the Pricing Authority started looking at medical devices
Price control was extended to devices, with the inclusion of two devices into the Drug Price Control Order, 2013; bare-metal stents and drug-eluting stents (cardiac stents)[21]. This was the direct result of a Delhi High Court Order[22].The writ petition sought the inclusion of coronary stents in the Essential Medicines List to control its price.
After this Order, the Pricing Authority conducted a study on the matter and released a report titled Report on Pricing of Stents[23] (‘Stent Report’). The Pricing Authority recommended that all types of stents be brought under price monitoring. This would prevent the price of stents from increasing by more than 10% annually. The Pricing Authority extended its powers toOrthopedic Implants (hip and knee)[24], citing protection of public interests[25].
The Stent Report also captured written requests received by the Pricing Authority, from various state and central government officials, to control and monitor prices of all the medical devices listed under the Drugs and Cosmetics Act, 1940[26]. In an earlier post, we demonstrated that medical device regulation depended on the device being listed under the Act. This meant that only devices that were “notified” by the Health Ministry, would be regulated for quality, sale, import, manufacturing, and clinical investigation. This has continued even after the release of the Medical Devices Rules, 2017 (‘MD Rules’), which also requires devices to be notified before they are regulated. However, the MD Rules did broaden the definition of medical devices and introduce risk classifications for these devices.
The present: recent developments and expanding scope:
In February 2020, the Health Ministry expanded the medical device definition to consider devices, including software, intended for the diagnosis, prevention, monitoring, treatment, or alleviation of any disease, disorder, disability, injury as a medical device[27]. This broadened definition means more devices can, in time, be regulated. It also means that as more devices get “notified”, the Pricing Authority will have more devices to price control and price monitor.
The Pricing Authority acknowledged the likelihood of this increased oversight in a meeting held in late January 2021[28]. In this meeting, the Pricing Authority decided to review the pricing information of the new devices that have been notified by the Health Ministry. The Pricing Authority is currently accepting information from the industries that manufacture or import four newly notified medical devices; namely, nebulizers, blood pressure monitoring machines, digital thermometers, and glucometers.
This additional layer of compliance could slow down India’s Atmanirbhar Bharat drive in healthcare, acting as a disincentive for an already compliance-heavy industry. The Commerce Minister recently highlighted how manufacturing costs for pharmaceutical products in India are lower than anywhere in the world, adding that innovation should ideally drive down the prices of pharmaceutical products[29]. If the aim is to increase access to affordable healthcare, now is the time to evaluate and harmonize all laws and policies affecting the medical devices industry’s ability to provide affordable healthcare. As shown in the table, carve-outs have been made, even if only for a few years, for other pharmaceutical products. Carve-outs that are time-bound can be considered for some notified medical devices that have been manufactured under schemes like the Production Linked Incentive Scheme.
Conclusion:
Last year’s medical device definition expansion, and the recent Pricing Authority’s meeting, indicate that as more devices get notified for regulation, they also run the risk of Pricing Authority oversight. A whole host of technology-based healthcare solutions could also come under the Pricing Authority’s scope, as these solutions get notified by the Health Ministry. This will be the case irrespective of whether they are imported or locally manufactured. If the Pricing Authority decides to look into a newly notified device, it would mean that in addition to complying with the requirements of the MD Rules and the Drugs and Cosmetics Act, manufacturers, and importers will also need to comply with the Drug Price Control Order of 2013. This also means that manufacturers and importers of these devices will have to interact frequently with the Pricing Authority, by reporting to it periodically. The medical device industry should engage with the Pricing Authority to build a meaningful relationship, and achieve a balance between the concerns of the regulator and the interests of the industry.
This article has been authored by Shambhavi Ravishankar with inputs from Anirudh Rastogi.
For more on the topic, please get in touch at contact@ikigailaw.com
Image Credits: Pixabay
[1] KPMG, Healthcare in India: Current State and Key Imperatives (Available at: https://assets.kpmg/content/dam/kpmg/in/pdf/2016/09/AHPI-Healthcare-India.pdf);https://www.nationalheraldindia.com/health/covid-19-pandemic-exposed-major-creeks-in-healthcare-india-needs-to-upgrade-critical-care-health-infra
[2] Constituted vide Government of India Resolution dated August 29, 1997, as an attached office.
[3] Delegation of Central Government powers to the National Pharmaceutical Pricing Authority (Available at: http://www.nppaindia.nic.in/wp-content/uploads/2021/01/Delegation-of-Powers-under-DPCO-2013-dt.-30.05.2013.pdf)
[4] Drugs (Price Control) Orders are released under Section 5 of the Essential Commodities Act, 1955.
[5] Drugs (Price Control) Order, 2013 (Available at: http://www.nppaindia.nic.in/wp-content/uploads/2018/12/DPCO2013_03082016.pdf)
[6] Schedule 1, (Available at: http://www.nppaindia.nic.in/wp-content/uploads/2018/12/DPCO2013_03082016.pdf)
[7] Drugs (Price Control) Order, 2013, Para 2(i)
[8] Drugs (Price Control) Order, 2013, Para 5
[9] Drugs (Price Control) Order, 2013, Para 2(b)
[10] Drugs (Price Control) Order, 2013, Para 6
[11] Drugs (Price Control) Order, 2013, Para 19. Fixation of ceiling price of a drug under certain circumstances.- Notwithstanding anything contained in this order, the Government may, in case of extra-ordinary circumstances, if it considers necessary so to do in public interest, fix the ceiling price or retail price of any Drug for such period, as it may deem fit and where the ceiling price or retail price of the drug is already fixed and notified, the Government may allow an increase or decrease in the ceiling price or the retail price, as the case may be, irrespective of annual wholesale price index for that year. (Available at: http://www.nppaindia.nic.in/wp-content/uploads/2018/12/DPCO2013_03082016.pdf)
[12] Drugs that are not listed in the DPCO are called “non-scheduled drugs”.
[13] Drugs (Price Control) Order, 2013, Para 20. Monitoring the prices of non-scheduled formulations.– (1) The Government shall monitor the maximum retail prices (MRP) of all the drugs, including the non-scheduled formulations and ensure that no manufacturer increases the maximum retail price of a drug more than ten percent of maximum retail price during preceding twelve months and where the increase is beyond ten percent of maximum retail price, it shall reduce the same to the level of ten percent of maximum retail price for next twelve months. (2) The manufacturer shall be liable to deposit the overcharged amount along with interest thereon from the date of increase in price in addition to the penalty. (Available at: http://www.nppaindia.nic.in/wp-content/uploads/2018/12/DPCO2013_03082016.pdf)
[14] Drugs (Price Control) Order, 2013, Para 22 and 23,
[15] Drugs (Price Control) Order, 2013, Para 21
[16] Drugs (Price Control) Order, 2013, Schedule II Form III
[17] Drugs (Price Control) Order, 2013, Para 29
[18] Para 2 of Drugs (Prices Control) Amendment Order, 2019. ( Available at: http://www.nppaindia.nic.in/wp-content/uploads/2021/01/6th-DPCO-2013-Amentment-dt-03.01.2019.pdf).
[19] Rule 2(1)(w), New Drugs and Clinical Trials Rules, 2019, (Available at: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/NewDrugs_CTRules_2019.pdf)
[20] Para 2 of Drugs (Prices Control) Amendment Order, 2019. ( Available at: http://www.nppaindia.nic.in/wp-content/uploads/2021/01/6th-DPCO-2013-Amentment-dt-03.01.2019.pdf).
[21] Ministry of Chemicals and Fertilizers, Department of Pharmaceuticals Notification S.O. 4100(E) dated December 21, 2016, http://dpco2013.com/files/data/dpcoamendments/3691482429292.pdf
[22] Shri Birendar Sangwan v. Union of India, Delhi High Court Order dated February 25, 2015 (Order available at: http://delhihighcourt.nic.in/dhcqrydisp_o.asp?pn=46567&yr=2015) – “treat this petition as a representation and pass an appropriate order in accordance with law within a period of three months from today.”
[23] SNational Pharmaceutical Pricing Authority, Report on Pricing of Stents (Available at: http://www.nppaindia.nic.in/wp-content/uploads/2018/08/REPORT-ON-PRICING-OF-STENTS04022016.pdf)
[24] Record of the discussions of the Authority on ceiling price fixation of knee Implants (48th meeting of NPPA concluded on August 14, 2017), http://dpco2013.com/files/data/authorityminutes201718/1871505542865.pdf
[25] Drugs (Price Control) Order, 2013, Para 19.
[26] Section 3(b) of the Drugs and Cosmetics Act, 1940.
[27] S.O. E. 648(E), February 11, 2020, https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NTU0OA==
[28] Department of Pharmaceuticals, Office Memorandum, Sub: Monitoring of MRPs of Medical Devices notified /regulated as ‘”drugs” under the Drugs and Cosmetics Act, 1940 and Drugs and Cosmetics Rules, 1945 (http://www.nppaindia.nic.in/wp-content/uploads/2021/02/NPPA-OM-on-Monitoring-of-MRPs-for-Medical-Devices-dated-16.02.2021-with-Annexures.pdf)
[29] https://indiaeducationdiary.in/piyush-goyal-addressed-the-6th-international-conference-on-pharmaceutical-medical-device-sector/